Update: See our article here regarding the final October 2020 Guidelines. On November 23, Innovative Medicines Canada and a number of pharmaceutical companies commenced an application for judicial review of the final Guidelines. See also the PMPRB’s November 20, 2020 webinar slides here. The definition of “Gap medicines” and compliance timelines were updated following a consultation (see article here), as were compliance timelines for Grandfathered and Gap medicines (see article here). A consultation is underway regarding a further update to the definition of Gap medicine, and other changes (see article here).
As previously reported , on June 19, 2020, the PMPRB released its much-anticipated revised draft Guidelines operationalizing the amended Patented Medicines Regulations , with comments due by July 20, 2020 (since extended to August 4, 2020). The backgrounder that contextualizes its revisions to the draft Guidelines was released on June 22, 2020.
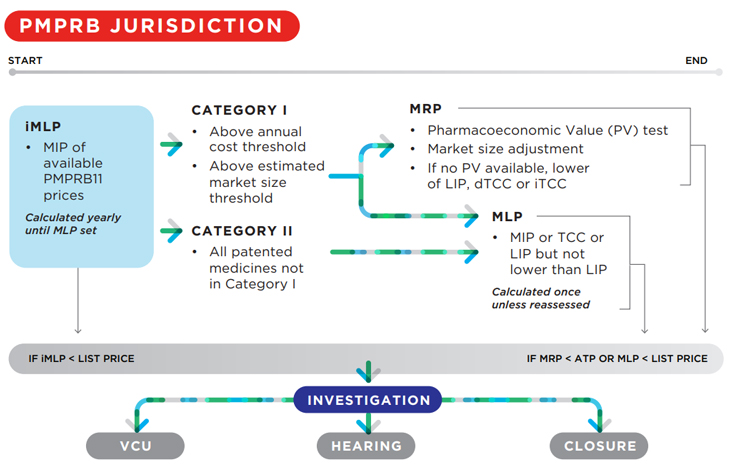
Generally, the revised Guidelines add complexity to the price review process for new patented medicines. Compare the schema in the initial draft Guidelines to the revised version:
2019:
2020:
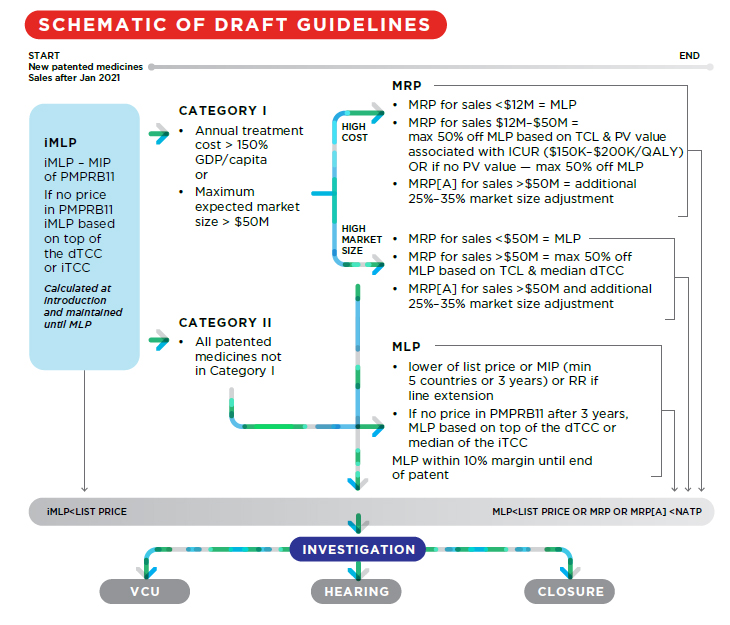
Notable changes include:
- Grandfathered, Line Extension, Gap and New Patented Medicines are now defined with new pricing frameworks;
- Category I thresholds for New Patented Medicines are increased;
- Therapeutic Criteria Levels are reintroduced; and
- Market size adjustment methodology is revised.
1. Grandfathered, Line Extension, Gap and New Patented Medicines Defined
In the first draft of the Guidelines, Line Extension and Gap products were not specifically addressed. The current draft now defines:
- Grandfathered patented medicines: dosage strengths and forms for patented medicines for which a DIN was assigned prior to August 21, 2019 and include dosage strengths and forms approved for new indications without a DIN change after August 21, 2019;
- Line Extensions of Grandfathered patented medicines are new dosage forms and strengths of Grandfathered patented medicines to which a DIN was assigned on or after August 21, 2019;
- Gap Medicines are patented medicines for which a DIN was assigned on or after August 21, 2019 and the first sale in Canada took place prior to January 1, 2021; and
- New patented medicines include all other dosage strengths and forms of patented medicines.
2. Revised price review process for Grandfathered medicines; new price review process for Gap medicines and Line Extensions
Under the initial draft Guidelines, the MLP of Grandfathered Medicines was set as the lower of (i) the median international list price for the PMPRB11 countries for which the patentee has provided information, or (ii) the price ceiling under the February 2017 version of the Guidelines.
Under the revised draft Guidelines, the MLP of Grandfathered Medicines and Line Extension medicines is set at the lower of:
(i) the highest international price (HIP) for the PMPRB11 countries for which the patentee has provided information; or
(ii) the patented medicine’s ceiling (e.g. the Non-Excessive Average Price (NEAP)) under the current Guidelines.
Under the revised draft Guidelines, the MLP of gap medicines is set as the lower of:
(i) the median international ex-factory list price (MIP) for the PMPRB11 countries for which the patentee has provided information; or
(ii) the patented medicine’s ceiling (e.g. the NEAP) under the current Guidelines.
The NEAP may be renegotiated for Grandfathered, Line Extension and Gap patented medicines “if the MLP is set by the patented medicine’s NEAP and if there is evidence that its calculation was uncharacteristically low due to the reporting of benefits, the patentee may submit a request for a higher MLP”.
Grandfathered, Line Extension and Gap patented medicines will be subject to a MLP but not to a Maximum Rebated Price (MRP).
3. Increased thresholds for Category I designation of New Patented Medicines
The new pharmacoeconomic factors will apply to Category 1 medicines. Under the revised draft Guidelines, fewer medicines will be categorized as Category I as both the market size threshold and per capita cost of treatment required to designate a medicine as Category I have been significantly increased. Under the initial draft Guidelines, a medicine would be designated Category I if either its market size threshold was $25M or its 12-month treatment cost was > 50% GDP per capita. Under the revised draft Guidelines, the market size threshold is $50M, and the 12-month treatment cost threshold is > 150% GDP per capita.
Notably, neither biosimilars nor generics will be designated Category I even if either of the above thresholds are met.
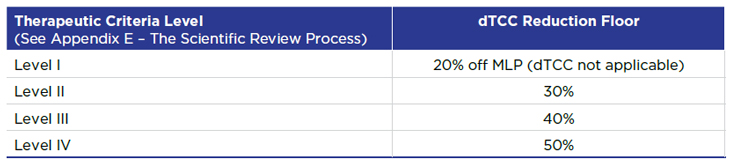
4. Reintroduction of Therapeutic Criteria Levels
As previously, the price of Category I medicines is capped by the Maximum Rebated Price (MRP). However, under the revised draft Guidelines, the MRP now takes into account a medicine’s Therapeutic Criteria Level (TCL). Levels of therapeutic improvement are a central feature of the current price review process.
- TCL I: first medicine sold in Canada that effectively treats a particular illness or effectively addresses a particular indication in a clinically impactful manner.
- TCL II: provides considerable improvement in therapeutic effect, relative to other medicines sold in Canada, in a clinically impactful manner.
- TCL III: provides a moderate absolute improvement in therapeutic effect, relative to other medicines sold in Canada.
- TCL IV: provides no or slight improvement relative to other medicines sold in Canada.
The TCL sets a price reduction floor depending on whether the relevant medicine has been designated Category I based on its market size, cost relative to GDP in Canada, or both.
As the MRP is now also a function of the TCL, which will be confidential, it should be more difficult to reverse engineer the MRP, addressing stakeholder concerns from the initial draft Guidelines (see Backgrounder).
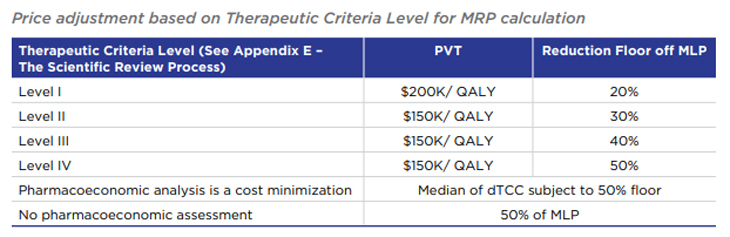
Medicines designated Category I based on cost relative to GDP
For medicines with a treatment cost exceeding 150% GDP per capita and (i) for which a cost-utility analysis is available and (ii) which have an estimated or actual maximum revenue greater than $12M per year, the TCL affects the Pharmacoeconomic Value
Assessment used to calculate MRP (see Appendix C). More specifically, the TCL dictates both the Pharmacoeconomic Value Threshold and sets a price floor relative to the Maximum List Price (MLP):
Medicines designated Category I based solely on market size
For medicines designated Category I based solely on market size and which have a market that exceeds $50M, the TCL sets the price floor for the domestic Therapeutic Class Comparison (dTCC) test used to calculate the MRP (see Appendix A):
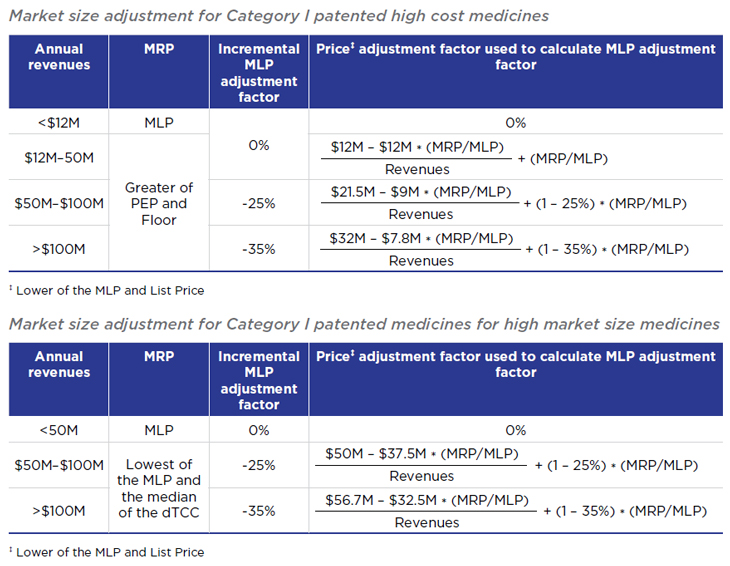
5. Modified market size adjustment methodology
The revised draft Guidelines collapse certain categories of market size adjustments, and create different adjustment methodology for medicines designated Category I based on cost and those designated Category I based solely on market size.
Under the initial draft Guidelines, medicines with annual revenues of $25-50M, $50-75M, $75-100M, $100-125M were subject to incremental adjustment factors of -10%, -20%, -30%, -40%, and -50%, respectively.
Under the revised draft Guidelines, no incremental MLP adjustment factor is applied until annual revenues exceed $50M, and the maximum incremental adjustment factor (for medicines > $100M), is 35%.
Concluding Remarks
Despite months of consultation, many questions remain unanswered in the revised draft Guidelines. For instance, the Guidelines do not speak to the content and form of information to be supplied—these and other important details are to be found in the Help section of the online filing tool, which is not yet publicly available. In addition, the revised draft Guidelines were not released with Case Studies, which can be important to understanding how the Guidelines will ultimately be applied.
Despite these uncertainties, patentees should nonetheless promptly consider the changes to the draft Guidelines and in particular, the impact on Grandfathered, Line Extension, Gap and New Patented Medicines. This information may inform submissions to the PMPRB during the consultation period.
Should you have any questions, please do not hesitate to contact a member of the Life Sciences Regulatory & Compliance Group.
Further Resources:
- New amendments to PMPRB pricing regulations push back deadlines by six months, June 10, 2020
- PMPRB releases draft Guidelines operationalizing the amended Patented Medicines Regulations, November 22, 2019
- Canada releases final amendments to Patented Medicines Pricing Regulations; in force July 1, 2020, August 18, 2019
The preceding is intended as a timely update on Canadian intellectual property and technology law. The content is informational only and does not constitute legal or professional advice. To obtain such advice, please communicate with our offices directly.
Related Publications & Articles
-
Finalized PMPRB Guidelines published
On June 30, 2025, the Patented Medicine Prices Review Board (PMPRB) published its new Guidelines for PMPRB Staff, which will come into force on January 1, 2026.Read More -
2025 mid-year highlights in Canadian life sciences IP and regulatory law
In the first half of 2025, the Rx IP Update team reported on a number of developments in Canadian life sciences IP and regulatory law. Below are our top stories.Read More -
Federal Court of Appeal confirms generic not required to address patent submitted before ANDS filing but listed after
On August 8, 2025, the Federal Court of Appeal (FCA) determined that the Minister of Health’s decision to list Canadian Patent No. 2,970,315 on the Patent Register eight days after it was submitted to...Read More